By disrupting immunological checkpoint (IC) expression, many hematological and solid tumors can evade the human body’s built-in antitumor immunity. Immune checkpoint (IC) inhibitors, a promising immunotherapy, re-regulates the activity of cytotoxic T lymphocytes and natural killer cells, re-establishing and enhancing innate immunity.
The FDA has approved treatments that target ICs like cytotoxic T-lymphocyte antigen (CTLA-4), programmed cell death (PD-1), and its ligand (PD-L1) to treat various types of cancers.1
Despite their potential, IC therapies are often non-response in most patients, a condition termed primary resistance. Other individuals who initially respond can later experience tumor relapse and acquire resistance, referred to as acquired resistance.2
Only a small portion of patients respond to IC inhibitor medication, thus restricting its clinical use. The emphasis has therefore moved to investigating the underlying mechanisms at fault and discovering new ICs or combination treatment approaches to boost therapeutic efficacy.
Immune checkpoint inhibitor resistance mechanisms
There are two types of IC inhibitor resistance mechanisms: primary and acquired. They cover immunological identification of cancer cells, cell signaling, gene expression, DNA damage response, and T-cell activation mechanism.3
There are numerous resistance mechanisms currently identified. This article explores the main mechanisms causing both primary and acquired resistances.
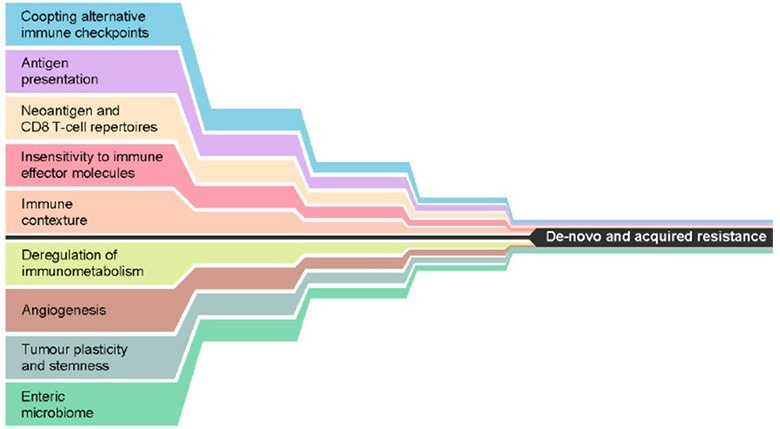
Figure 1. Mechanisms that may, either alone or in combination, lead to de novo or acquired resistance to immune checkpoint inhibition. Reproduced from Fares, et al.3. Image Credit: ACROBiosystems
Immune contexture
Immune contexture, also known as the tumor microenvironment, refers to immune factors extrinsic to cancer cells (immune and stromal cells, cytokines, and other biologics that influence therapeutic response). This microenvironment’s altered molecular and cellular compositions create an immunosuppressive atmosphere.
Regulatory T cells (Tregs), myeloid-derived suppressor cells, and tumor-associated macrophages all contribute to this modification through various methods, including cytokine secretion, T effector cell suppression, and elevated transforming growth factor beta. This directly affects the primary response to IC inhibitor medication.
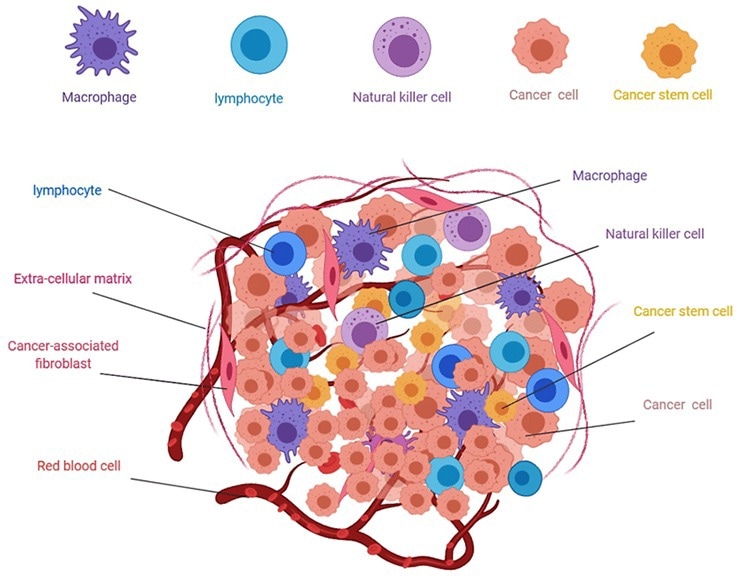
Figure 2. Composition of solid tumors4. Image Credit: ACROBiosystems
Co-opting alternative immune checkpoints
The evolution of response, a process frequently altered in tumor malignancies, is a crucial component of the innate immune system. In contrast to the tumor microenvironment, which fosters an immunosuppressive environment, the altered immune response prevents T cell proliferation and diversification.
Multiple immunological checkpoints co-expressed together lead to a severely exhausted T-cell state that impairs effector function, causes progressive T-cell function loss, alters transcriptional states, and promotes antigen persistence.
Targeting or co-targeting these alternative checkpoint receptors could be a way to prevent acquired resistance in situations where immunological checkpoints are overactive and co-expressed.
Tumor immunogenicity
Tumor immunogenicity, also known as sensitivity to immune effector chemicals, is the last resistance mechanism to IC treatments. The number of immunogenic neoantigens perceived as foreign or tumor mutational burden (TMB) can be used to quantify tumor immunogenicity.
Immune system-interacting heterogeneous malignancies more frequently choose low TMB cancers. According to a study by Anagnostou et al. on recurrent non-small-cell lung carcinomas (NSCLC), this mechanism plays a significant role in acquired resistance.4
Novel immune checkpoint targets for immunotherapy
The importance of ICs in directing the innate immune system is evident in light of the revelations mentioned above. The development of the immune suppressive TME and altered immune response evolution can be attributed to the over- and under-expression of ICs.
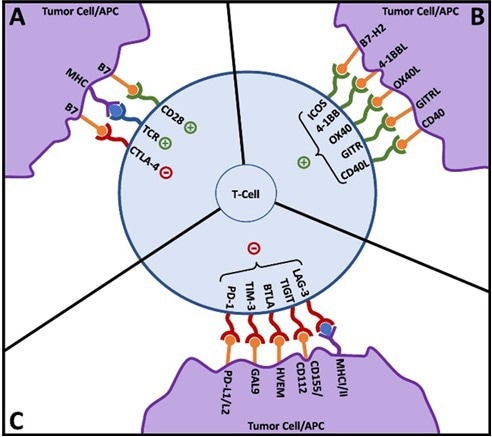
Figure 3. T-cell activation and co-signaling. Reproduced from Fares, et al.3. Image Credit: ACROBiosystems
The intricacy of IC inhibitor resistance and the existence of unknown mechanisms is revealed by the diversity in responsiveness to IC inhibitor therapy.
The development of new IC inhibitor therapies, therefore, holds the promise of overcoming resistance mechanisms and extending the current immunotherapies’ restricted applicability.
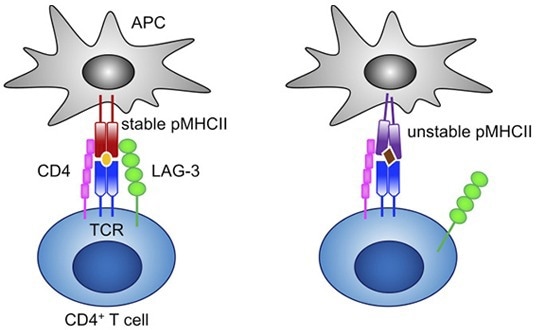
Figure 4. LAG-3 selectively binds to stable pMHCII and inhibits the activation of CD4+ T cells that recognize stable pMHCII.6 Image Credit: ACROBiosystems
LAG-3 is a well-known IC inhibitor. LAG-3 is a significant immunosuppressive molecule that inhibits T cell cytokine synthesis, CD4/8 expansion and promotes Treg adoption to stop autoimmune disease and tissue damage.5
Blocking LAG-3 enhances other IC inhibitors while causing a positive immunological response against tumor cells. Clinical studies have shown that combining LAG-3 with PD-1 therapy enhances the therapeutic impact, but LAG-3 monotherapies only provide a mild response.
A LAG-3/PD-L1 combination therapy (Opdualag) for metastatic or unresectable melanoma received FDA approval in March 2022.8 LAG-3 inhibitors are currently being tested in more than 100 clinical trials, underscoring its promise as an IC treatment.
TIM-3 is another possible IC target. Numerous immune cells, including CD4/8 T cells, Tregs, myeloid cells, NK cells, and mast cells, express TIM3.9 The cellular variety of TIM-3 leads to the control of immune response via several cellular pathways.
Although the pathophysiology of TIM-3 in innate immunity is still unknown at this time, its function in numerous cellular pathways and the substantial preclinical data suggest TIM-3 as an immune checkpoint, making it a prospective immunotherapy therapeutic option for conditions other than cancer.
Perspectives on immune checkpoint therapies
Despite the tremendous potential of immunotherapy, primary or acquired resistance is frequently present, and the use of IC inhibitor therapies is restricted due to their specific purposes.
This is partly due to a lack of knowledge on immune checkpoint and resistance processes and a dearth of therapeutically beneficial immune checkpoint targets.
Notwithstanding the clinical and biological knowledge gaps, immunotherapies’ early encouraging outcomes and long-lasting effects cannot be disregarded. Immunotherapy has tremendous potential for revealing the mechanisms behind the human body’s innate defensive mechanisms.
ACROBiosystems is dedicated to creating and upgrading several IC proteins, inhibitor screening kits, and overexpression cell lines to hasten the creation of innovative immune therapeutics.
Image Credit: ACROBiosystems
References
- Archilla-Ortega, A., Domuro, C., Marin-Liberal, J. et al. Blockade of novel immune checkpoints and new therapeuic combinaions to boost anitumor immunity. J Exp Clin Cancer Res 41, 62 (2022).
- Bagchi S, Yuan R, Engleman EG. Immune Checkpoint Inhibitors for the Treatment of Cancer: Clinical Impact and Mechanisms of Response and Resistance. Annu Rev Pathol. Jan 24;16:223-249. (2021)
- Fares CM, Van Allen EM, Drake CG, Allison JP, Hu-Lieskovan S. Mechanisms of Resistance to Immune Checkpoint Blockade: Why Does Checkpoint Inhibitor Immunotherapy Not Work for All Paients? Am Soc Clin Oncol Educ Book. Jan;39:147-164. (2019)
- Ghmkin H., Seno M. Blood and Cancer: Cancer Stem Cells as Origin of Hematopoieic Cells in Solid Tumor Microenvironments. Cells 9,1293 (2020)
- Anagnostou V, Smith KN, Forde PM, et al. Evoluion of neoanigen landscape during immune checkpoint blockade in non-small cell lung cancer. Cancer Discov. 7:264-276 (2017)
- Maruhashi T., Okazuki T., et al. LAG-3: from molecular funcions to clinical applicaions. J. Immunother Cancer. 8(2):e001014 (2020)
- Marin-Acevedo, J.A., Kimbrough, E.O. & Lou, Y. Next generaion of immune checkpoint inhibitors and beyond. J Hematol Oncol 14, 45 (2021).
- Food and Drug Administraion. FDA approves Opdualag for unresectable or metastaic melanoma (2022).
- Wolf, Y., VK Kuchroo, et al. TIM3 comes of age as an inhibitory receptor. Nature Reviews Immunology 20, 173-185 (2020).
About ACROBiosystems
ACROBiosystems is a cornerstone enterprise of the pharmaceutical and biotechnology industries. Their mission is to help overcome challenges with innovative tools and solutions from discovery to the clinic. They supply life science tools designed to be used in discovery research and scalable to the clinical phase and beyond. By consistently adapting to new regulatory challenges and guidelines, ACROBiosystems delivers solutions, whether it comes through recombinant proteins, antibodies, assay kits, GMP-grade reagents, or custom services. ACROBiosystems empower scientists and engineers dedicated towards innovation to simplify and accelerate the development of new, better, and more affordable medicine.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.