Induced pluripotent stem cells (iPSCs) represent a significant breakthrough in the fields of cell therapy and regenerative medicine.
The distinct ability of iPSCs to differentiate into practically any human cell type opens up new pathways for the development of innovative allogeneic cell therapies designed to treat a wide range of diseases, for example, neurological diseases, cardiovascular diseases, ophthalmological diseases, metabolic disorders, and cancers.
Engineered iPSC-derived immunotherapy has also emerged as a promising avenue for the treatment of autoimmune diseases and cancers.
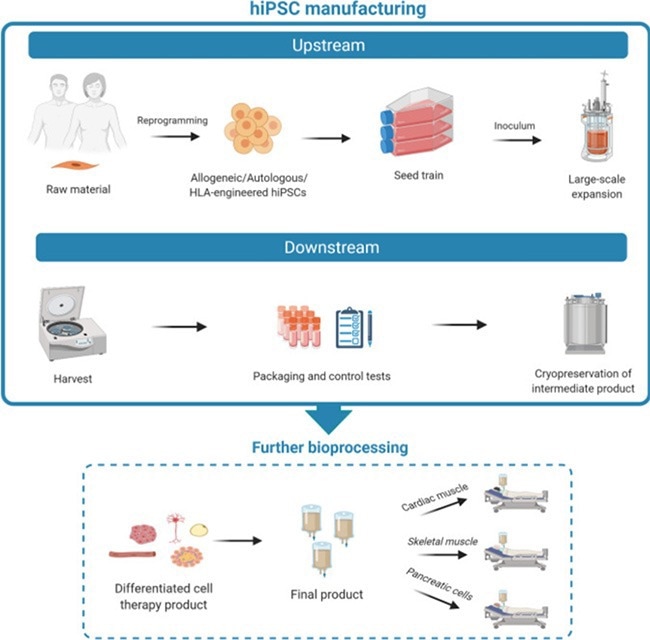
Figure 1. illustrates the manufacturing process of iPSCs encompassing both upstream and downstream phases. Additionally, subsequent bioprocessing steps are depicted, crucial for the refinement and generation of various functional, differentiated cell types. Image Credit: ACROBiosystems
The clinical application of iPSC technology faces a number of challenges due to the delicate nature of these cells and their reliance on a supportive extracellular matrix environment to achieve efficient expansion.
When it comes to the manufacture of iPSCs, the process involves both upstream expansion and downstream differentiation phases. These are followed by further bioprocessing phases to produce functional, differentiated cell products (Figure 1).
While the differentiation of iPSCs is typically well defined, the implementation of scalable upstream iPSCs expansion that meets the required regulatory guidance for clinical use continues to pose a challenge.
Significance of extracellular matrix and laminin for iPSC
The quality and yield of iPSCs during expansion are significantly impacted by the extracellular matrix employed in the facilitation of cell adhesion and proliferation.
Over the past ten years, there have been major advances in the optimization of specialized culture media and matrices designed specifically to accommodate iPSC culture.
To achieve scalable, clinical-grade production of iPSCs from biological donors, an ideal matrix should serve as an effective yet consistent scaffold for cell adhesion and proliferation while preserving pluripotency.
Laminin is a large adhesive glycoprotein composed of heterotrimers of α, β, and γ chains. This protein has become central to the extracellular matrix and is designed to support the undifferentiated expansion of iPSCs and embryonic stem cells (ESCs).
A range of different laminin combinations can be used, each exerting distinct functions in different tissues. For example, these functions may involve basement membrane construction and regulation of cell adhesion, migration, proliferation, and differentiation.
There are currently at least 16 known laminin isoform combinations, each performing a vital function in cell growth and development.
Integrin receptors and iPSC adhesion
Laminin’s extraordinary potential to support iPSC culture stems from its ability to bind with a wide range of cell surface receptors, especially integrin receptors. Integrin α6β1 is highly expressed on the surface of iPSCs and ESCs, with laminin isoforms such as laminin-511, -332, and -111 displaying a high binding affinity for integrin α6β1.
Functional blocking studies have also determined that disrupting this integrin α6β1-laminin interaction hinders cell adhesion, serving to further highlight the key role of this receptor in mediating laminin-dependent cell adhesion.
ACROBiosystems’ laminin products
In order to meet the increasing demand for consistent, high-quality laminin matrices suitable for stem cell research, ACROBiosystems offers a wide range of recombinant laminin proteins.
The company’s protein offering includes GMP-grade and premium-grade Laminin 521, premium-grade Laminin 511, and a range of other laminin proteins specifically tailored for iPSC culture applications.
All of ACROBiosystems’ products are subject to rigorous quality control and validation to ensure consistent performance in terms of their iPSC adhesion, proliferation, and maintenance of pluripotency.
These proteins have also been specifically developed to meet the strict safety and scalability requirements that are commonplace in high-end clinical and research applications.

Figure 1. Laminin 521 (GMP-LA5H24) effectively maintains the expansion of human iPSCs. Image Credit: ACROBiosystems
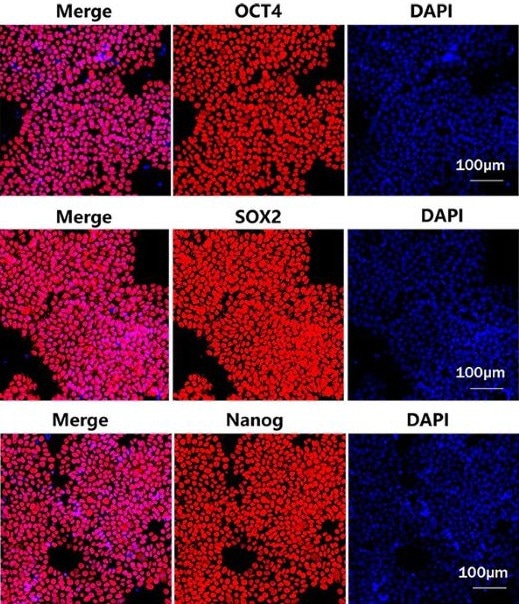
Figure 2. Laminin 521 (GMP-LA5H24) could maintain the stemness of iPSC after several passages. Image Credit: ACROBiosystems
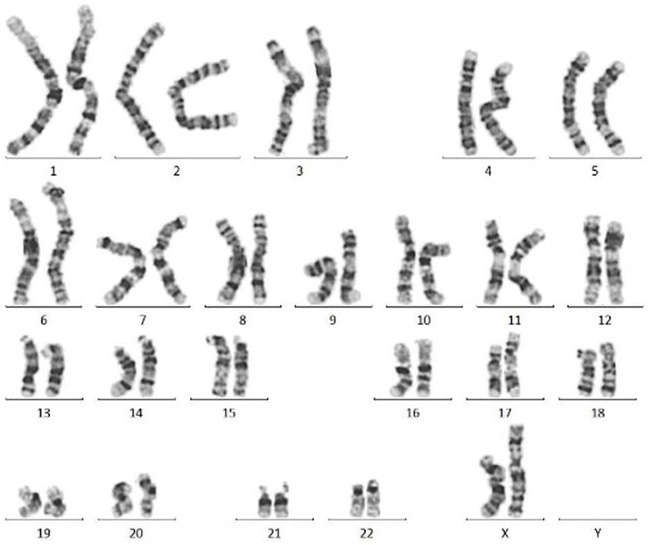
Figure 3. Normal karyotype (46, XX) was found in hiPSCs with Laminin 521(GMP-LA5H24) coating after 10 passages. Image Credit: ACROBiosystems
Acknowledgments
Produced from materials originally authored by ACROBiosystems.
About ACROBiosystems
ACROBiosystems is a cornerstone enterprise of the pharmaceutical and biotechnology industries. Their mission is to help overcome challenges with innovative tools and solutions from discovery to the clinic. They supply life science tools designed to be used in discovery research and scalable to the clinical phase and beyond. By consistently adapting to new regulatory challenges and guidelines, ACROBiosystems delivers solutions, whether it comes through recombinant proteins, antibodies, assay kits, GMP-grade reagents, or custom services. ACROBiosystems empower scientists and engineers dedicated towards innovation to simplify and accelerate the development of new, better, and more affordable medicine.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.